Further Catalysts to Knowledge: Learning the Basics
Robert Boyle
The laws of Thermodynamics were discerned by studying the relationships between the four parameters that dictate the physical behaviour of matter - especially regarding matter in its gaseous state. Those four variables are: pressure; volume; temperature and moles, or the amount of material being studied. Long before the science of thermodynamics truly got under way, these same principles were studied and applied in other ways. In fact, this is how Robert Boyle (25 January 1627 - 31 December 1691) whose first scientific love was chemistry, made his name and came to be known as the father of Chemistry. Boyle had many accomplishments in his career, including creating litmus paper, correctly explaining that sound propagated through air: succinctly stating: "Sound consists of an undulating motion of the air." His further accomplishments ranged from identifying and explaining why ice is less dense than water and would thus float on water, to describing the specific and relative gravities of different substance (sometimes called their specific densities). Specific and relative gravities allow one to calculate mathematically, which substances are denser than others when compared to a reference material. More than that, he proposed the existence of and named elements, explaining their nature in his book The Sceptical Chymist:
And, to prevent mistakes, I must advertize you, that I now mean by elements, as those chymists that speak plainest do by their principles, certain primitive or simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the ingredients of which all those called perfectly mixt bodies are immediately compounded, and into which they are ultimately resolved" Robert Boyle
The current definitions of elements, mixtures and compounds followed. In those definitions, you will not fail to notice, and I am sure be greatly impressed by the exactness of Boyle's understanding of the fundamental nature of these entities. This 'fundamental nature' was the source of much debate before Boyle, some saying atoms where indivisible and another camp known as Corpuscularianism thinking the fundamental building blocks of nature were divisible. In his aforementioned work The Sceptical Chymist, Boyle threaded a thin line that wove the two competing views into a more explanatory whole. But we are not celebrating him for any of those reasons. Boyle finds inclusion in our discussion for his success in experimentally deriving his eponymous gas law. Wikipedia states the law thus:
The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system" Boyle's Law - Wikipedia
Again in plain English, the parameters of volume and pressure are oppositely related - like the seats on a see-saw. When the volume of the gas' container increases, the pressure goes down: AND when the pressure goes up it's because the volume of the container has decreased, as long as the temperature and the amount of gas stays the same! Written out mathematically it looks like ths:
P & 1/V Meaning Pressure is inversely (see-saw) related to the volume
or
PV = k Meaning Pressure multiplied by the Volume equals some constant k
That fact is significant. The fact that the product (the result of multiplying one thing by another) of the pressure and volume of a gas is constant as long as the temperature and amount of gas, remain the same - for:
So long as temperature remains constant the same amount of energy given to the system persists throughout its operation and therefore, theoretically, the value of k will remain constant" Boyle's Law - Wikipedia
As simple as this formula seems, its significance is earth shattering. As I have highlighted before it is not the fancy formulas that underpin and govern physical reality but the simple things that matter the most. Wax on. Wax off, Danielson. Wax on, wax off ... your diligence in getting to grips with the foundations of reality will soon pay off. Keep focused. Stay the course. Persevere until you start to understand; then you will not be able to stop the avalanche of clarity - even if you wanted to!

Robert Boyle, was another in a long list of top tier scientists who believed that reason was the common denominator in both religion and the sciences. As such, such scientists were not afraid of evidence and/or experiment, nay, they see them as their greatest assets in proving the existence of God - and his Creatorship of the universe. For Boyle, the only way to truly follow the disciple of science was to "keep his judgement as unprepossessed as might be, with any of the modern theories of philosophy, until he was 'provided of experiments' to help him judge of them." In other words, he didn't entertain theories, he considered only evidence as produced by experiment. Wikipedia, continues, "Nothing was more alien to his mental temperament than the spinning of hypotheses." Do you get the picture? Men of Science, who believe in God maintain a strict discipline of focusing their thoughts only on evidence, experiment and reason, and show a strong aversion for hypothesis, or unfounded theories. An interesting side-note is that the greatest discoveries in the history of science are due to these kind of individuals, men like Copernicus, Kepler, Galileo, Boyle, and Newton.
Establishing the Ideal Gas Law
Boyle's great achievement would prove instrumental in shaping the field of thermodynamics which would soon burst onto the scene. His law, together with laws from other innovators like Jacques Charles (Charle's Law), Joseph Louis Gay-Lussac (Gay-Lussac's Law) and Amedeo Avogadro (Avogadro's Law) helped to establish the Ideal Gas Law as we know it today. Recall that we said that the laws of thermodynamics were discovered after scientists discerned the relationships between four parameters that are critical to the control and understanding of physical matter. Once again, those parameters are: pressure; volume; temperature and moles, or the amount of gaseous material being studied. What each of the gentlemen just listed did was separately discover the different relationships to be found between those four parameters. For instance, while Boyle, discovered the relationship between pressure and volume; the relationship between volume and temperature was discovered by Charles and is called Charles' Law. Likewise, Gay-Lussac discovered the relationship between pressure and temperature. So each scientist uncovered a different aspect, or relationship, of the same overall system. Taken together, these discoveries were grouped into what became known as the combined gas law. These three laws were in turn united with Avogadro's Law to form the ideal gas law. Lastly, a few words about the significance of Avogadro's Law. This law proved indispensible to the practical everyday work of chemists in conducting their experiments. Remember that one of the parameters is the amount of gas present, known as its 'moles' - or molecules. Avogadro's Law is useful in defining the term 'molecules.' Wikipedia stated is current definition as:
Avogadro's law states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules
For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant" Avogadro's Law - Wikipedia
Avogadro's Law was a way of standardizing our understanding of gases and our ability to experiment with them. It meant that, although different gases are made up of particles (atoms) of different sizes from each other - for instance an oxygen atom is not the same size as an atom of hydrogen - as long as two samples of the different gases had the same volume as each other, and at the same temperature and pressure: then they would both contain the same amount of molecules. How fantastic. Now, how do molecules relate to atoms? Molecules are a neutrally charged group of two or more atoms. Its that simple. The conditions necessary for Avogadro's Law to be true are important enough to be stated again, for clarity: all three of the other 'parameters,' pressure, volume and temperature have to be constanst. A working example of the principle at work will help to make its importance clear to all.
Equal volumes of gaseous hydrogen and nitrogen contain the same number of atoms when they are at the same temperature and pressure, and observe ideal gas behavior. In practice, real gases show small deviations from the ideal behavior and the law holds only approximately, but [it] is still a useful approximation for scientists" Avogadro's Law - Wikipedia
An ingenious graphic of how the contributions of each scientist helped to define the whole is provided below in Figure 23.
Laws of nature are discovered when the hidden relationships between different variables, or entities are uncovered. In the case of the Gas law, these discoveries were made by three different contributors. This fantastic graphic in Figure 23 illustrates who discovered what law - and, which variables the law regulates. The mapping of these relationships is colour coded. For instance Gay-Lussac discovered the relationship between pressure and temperature (in green). Avogadro, on the other hand, discovered the hidden relationship between the volume of a gas and the amount of gaseous substance present, represented by the letter N - for the number of moles (a way to measure gases) present. In the illustration, this relationship is represented by the orange colour, its discover's name is also in orange. You can follow the same pattern to confirm what variables Charles and Boyle discovered.
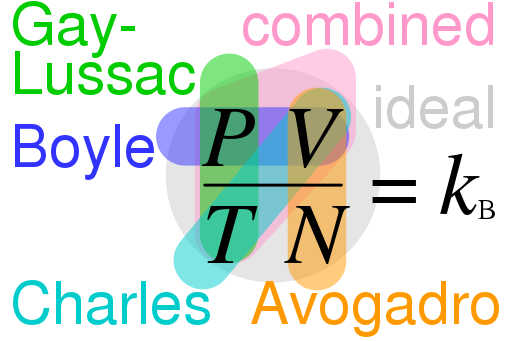
As for the ideal gas law: it is the result of putting the three hitherto discussed incremental discoveries - the 'combined gas law' - together with Avogadro's Law. So the ideal gas law is the combination of three experimentally proven insights into how gases work, with one not perfect but close approximation of the nature of gases as a whole. One that is close enough, that scientists use it freely and extensively in practice. The ideal gas law is thus, itself:
A good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: P V = n R T where P, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It is the same for all gases. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857" Ideal Gas Law - Wikipedia
From Terra Pingus to Phlogiston to the Caloric
Sometimes when we glance backward at the progress of science we cannot help but be stupefied by the seemingly ridiculous explanations former generations had for the many phenomena found in nature. The truth though, is that when it comes to falsehoods all generations just recycle the same thought pattern, updating only its technical setting to be in lock-step with the current age. So the explanations change according to whether the setting is agricultural, industrial, or digital. Is there a difference between the Ephesians - living in an agrarian age - believing the statue of Artemis fell from the sky and the current craze for believing UFO's are being hidden from the public by the American government at area 51? Same plot, different setting. It is important to realize that humans who are currently living are not superior to the Ephesians who believed in Artemis. The Ephesians were not apemen. Ephesus was a prosperous trading center full of educated individuals. Mankind has always been just as you are today. The origin of our kind is not Lucy, but Adam, as such mankind has been the same, throughout our history! As for Lucy, that's a story for another day. For now we focus on the topic at hand.
Johann Becher
The theory of Phlogiston is just one example of circular reasoning running in endless loops. Phlogiston, was supposedly a fire-like fluid dreamed up in 1667 by an intellectual called Johann Joachim Becher (6 May 1635 - October 1682), as a way of explaining combustion and other chemical reactions, including rust. It was later formalized into its final form by George Ernst Stahl. For more than 2 000 years, from times predating Aristotle, people had imagined all things in nature to be composed from four essential elements: earth; water; fire and air. However, Becher asserted in his most famous book, Physica Subterranea, that fire should be removed from the then accepted catalogue of four elements and replaced with an extended five-range catalogue of earth, now comprised of three new sub-types of earth, namely: terra lapidea, terra fluida, and terra pinguis, together with water and air. According to him, all matter was the combination of these five elements. Becher's proposal was that, what we would now call a periodic table, was actually only made of these five elements and was sufficient to explain all the variety seen on earth, and its everyday processes. As always, the embedding of such fanciful ideas into the mainstream of society is a multiple step process that gains strength with time, even with the absence of any evidence to substantiate its lofty claims. And so it proved with the foundations of phlogiston theory. The torch would now be passed to Becher's student, the aforementioned Stahl, upon whom Becher had great influence. Becher had provided only the rough outline of phlogiston theory, it was Stahl who would flesh out its final form. In the end, Becher's contribution was only to define his various terras. The difference between these assorted 'earths' was, they said, the way they reacted chemically. He defined terra pinguis - our main point of focus - as the combustible earth. He imagined all combustible substances to contain this ignitable matter "terra pingus." To make it clear, a piece of wood didn't burn because it was terra pinguis, but rather because it contained terra pinguis and once all the terra pinguis had been burned off, what was left - the ash - was classified as terra lapida.
In this scheme, air was the medium through which the earth and water could mix and generate all the compounds responsible for the variety we see around us. Of course, "air" was an umbrella term for what we now call gases. So you didn't have carbon or oxygen, for instance - they had not yet been discovered - rather, you had different kinds of "airs" that had fanciful names such as inflammable air, sugar of Saturn, and vitriol of Venus. They inturn, were classified by how they influenced different chemical transformations. Becher, was an alchemist not a chemist. You can understand how that influenced his approach to science because he famously believed that with the right mixture of alchemical materials, he could turn himself invisible! It was upon his ideas that scientists early in the 1700s, based their perceptions of how chemistry works - good luck with that.
One reason Becher's wildly inaccurate postulations about the nature of reality were so readily accepted is that he made them in the days when there was no discernable difference between chemistry and alchemy. This began to change 21 years before his death with the 1661 publication of The Sceptical Chymist by Robert Boyle. Alchemy would thus die a natural death with the passing of Becher's generation of scientists who held on to the misinformed beliefs with all their might. However, it would take longer for the effects of his ideas about chemistry, itself, to be overturned. For this reason and others, the belief in phlogiston became so widespread and embedded in the reasoning of that age that it endured for well over a century, despite the fact that there was zero evidence to support its existence or assumed dynamics. As Hank of Crash Course says:
Led by the prominent English Chemist, Joseph Priestley, these old-timers kept modifying Phlogiston theory so that it could rationally account for chemical reactions without falling apart, due to the whole phlogiston-in versus oxygen-out thing. Well into the 1780s, many chemists still believed in phlogiston - which no one had actually seen or measured - simply because it was familiar" Hank Green - The New Chemistry #18

Just like good science is based on the scientific method and its reliance on experiment, and evidence, bad science is based on reliance on ethers, whose properties are dreamed up by their latest advocates. As we will show, each period in human history from the era of Aristotle onwards, has had a leading "scientist" who proposed some version of the world, based on an ether. These ethers, morph in-line with the age in which they are proposed. Indeed, we will discover that at no time in the millennia long history of Science, has there been a period when ethers were not proposed and adopted as leading candidates for explanations of how the universe works. For his part, Johann Becher proposed Phlogiston theory. Two years later, in 1669 he published Physica Subterranea, for which he gained the widest acclaim. The book being said to be "above reproach." Perhaps, it was due to his growing prestige that his Phlogiston theory caught on, and soon became established "science." In the end, it took more than a century to dislodge a theory that a minimum amount of critical analysis at the beginning would have shown didn't merit any consideration!
George Ernst Stahl - How Terra Pingus Became Phlogiston
Phlogiston's wide acceptance was no coincidence. There was a large list of prominent scientists who staked their reputations on the validity of the theory and thus helped it gain wide acceptance, both among other scientists, and more generally, in the wider public sphere. We list a few of them in detailing the rise of phlogiston theory in eighteenth and nineteenth century Europe. We start with George Ernst Stahl (22 October 1659 - 24 May 1734), whom Wikipedia credits with renaming Becher's "terra pingus" to "phlogiston." When Stahl was a professor of chemistry at the Martin Luther University of Halle-Wittenberg in Germany, he formalized the theory of phlogiston - the term had been around since about 1606. Stahl simply popularized it by formalizing its definition, writing about it in his 1697 publication Zymotechnia Fundamentalis. Stahl imagined that combustible or chemically reactive materials (ones that could undergo oxidation) contained phlogiston, a fire-like element and were thus "phlogisticated." When all the phlogiston in any given material had been transferred to the surrounding air, the material was burned up and now existed in a dephlogisticated state. Regular air in an open-air environment could absorb all the transferred phlogiston you could throw at it and thus allowed the burning of many items simultaneously (think of a forest fire). It gets better. In the opposite scenario, where a flame is enclosed under a glass the flame would go out due to the limited amount of air within the enclosure. Since there was limited air, there was a limited amount of phlogiston that could be transferred and thus such flames would quickly go out, since the limited amount of air, would quickly get saturated with phlogiston, arresting any further combustion. The melding of Becher's ideas on the different terras with Stahl's updated phlogiston theory made perfect sense to him. Since phlogiston transferred from substances into the surrounding air in combustion, once materials had been burned up they were free of phlogiston - they were "dephlogisticated" - and the air, was now phlogisticated. However, phlogiston in air could not burn, only if it was found in material substances, hence the phlogiston in air was transferred into plants and other parts of nature. Stahl, kept refining his definition until in 1723, when he published yet another work on chemistry called Fundamenta Chymiae. Wikipedia describes his views:
According to Stahl, phlogiston was a substance that was not able to be put into a bottle, but could be transferred nonetheless. To him, wood was just a combination of ash and phlogiston .... He did not account for the increase in weight on combustion of tin and lead that were known at the time" Wikipedia on George Stahl
Stahl, for his part, at least conducted experiments to quantify the claimed properties of Phlogiston. Before him, there was no experimental evidence of, or for Phlogiston - just Johann Becher's word. Of course, after him, there was still none - but at least he tried to substanstiate his beliefs! Imagining phlogiston, to be a substance that filled all combustible materials, Stahl set about performing experiments with metals and other substances, that would separate the phlogiston, from the materials it was to be found in. This led him to believe that all flammable materials were made of two elements, calx, or ash, and phlogiston. (This was a clever, but wrong guess.) Materials with both elements could catch fire, but materials with just the ash, were dephlogisticated, and thus couldn't burn. Notice the tell-tale sign that you're dealing with an idea based on an ether and not one founded in empirical evidence: descriptions of Ethers, work on describing how things look, and never how on the emprical data of how they work! Such descriptions always focus on appearances, never empirical evidence. You will see this pattern again.

The line of influencers continued without letup, all from the same school of thought - literally. John Heinrich Pott, was a student of one of Stahl's students and he was the next one to try and advance phlogiston theory. By the time he came on the scene there were many problems his contemporaries had identified in phlogiston theory. The problem was, each new scientific discovery uncovered more and more issues that phlogiston theory could not explain. Instead of the new evidence offering verification through proof, it was highlighting more and more inconsistencies of the failing theory! The cracks were getting bigger and phlogiston theory was in full-on crisis mode. One of the most notable cracks was the "mass" problem. Since phlogiston was proposed to be an ignitable substance inside combustible or chemically reactive substances, when such reactions occurred including fire, and rusting, there should have been less phlogiston and hence, less mass in the substance after the reactions than before. However, the opposite was true. For instance, when metals rusted, they were heavier (more massive) afterwards than before. What was going on? Pott's contribution to phlogiston theory was not to resolve its growing inconsistencies, but to help entrench it as the conventional wisdom of the day. To that end, he aimed his efforts at the general public and used less technical examples in describing what phlogiston was and how it supposedly worked. In this way he made the descriptions of phlogiston easier to grasp, but did nothing for explaining how it supposedly worked! He compared phlogiston to light and fire, something lay people could easily relate to. And just like light and fire, Pott claimed, phlogiston was easy to understand, but hard to define.
So hard was it to define, that Pott argued vigorously that phlogiston should in fact not be considered to be a particle at all. Rather, he forcefully asserted that phlogiston should be considered to be an essence that permeates substances as: "in a pound of any substance one could not simply pick out the particles of phlogiston. Phlogiston permeating everything in the universe, it could be released as heat when combined with acid." (Wikipedia). It was hard to define argued Pott, because though phlogiston was a part of all substances, it was not an easily identifiable particle within them. No one could take a sample of some substance and then identify the phlogiston within it. Thus, Pott promoted the idea that phlogiston should be thought of as an essence that permeates all substances. In other words - an ETHER! Wikipedia records that it was believed that, "Phlogiston permeated everything in the universe." Below, are some tenets of Phlogiston theory as promoted by Pott:
- The form of phlogiston consists of a circular movement around its axis
- When homogeneous it cannot be consumed or dissipated in fire
- The reason it causes expansion in most bodies is unknown, but not accidental. It is proportional to the compactness of the texture of the bodies or to the intimacy of their constitution
- The increase of weight during calcination is evident only after a long time, and is due either to the fact that the particles of the body become more compact, decrease the volume and hence increase the density as in the case of lead; or that little heavy particles of air become lodged in the substance as in the case of powdered zinc oxide
- Air attracts the phlogiston of bodies
- When set in motion, phlogiston is the chief active principle in nature of all inanimate bodies
- It is the basis of colors
- It is the principal agent in fermentation
I am sorry to put you through that, but I can't suffer alone. It's not right. None of Potts' proposals had any experimental backing, they were just products of his imagination. As Wikipedia puts it:
Potts' formulations proposed little new theory; he merely supplied further details and rendered existing theory more approachable to the common manPhlogiston article - Wikipedia
Like alchemy, phlogiston theory has long since been discredited. The review of phlogiston theory is useful to us not because it sheds light on the workings of nature, but precisely because it was wrong about how the universe is constituted and how it operates. Important to note is the fact that none of the reasons for it were based on facts, only imagination. There's a lesson in there for all of us! Another lesson we can glean is in how phlogiston theory met its demise! Its proponents argued that phlogiston (formerly terra pinguis) is what enabled materials to burn. Once a substance had burned out, it no longer fell under the Terra Pinguis category, but was now considered to be Terra Lapida. Also, the amount of phlogiston determined how combustible an element or substance was - the name literally meant burning up! The only problem is that it was disproven by the very process that it was trying to describe - what later became known as oxidation! Let us take a moment to go through this problem. It is important for our overall understanding of what true Science is and how it differs from mere imagination. Oxidation (rust) is actually only one-half of two simultaneous and spontaneous chemical reactions: 1)REDuction and 2)OXidation that are together known as a REDOX reaction. Redox reactions occur when an element, say iron (chemical symbol Fe), gives up its electrons to another element. The element which loses electrons is always said to be oxidized. In the case of rust, the second element, the one that accepts the electrons is oxygen. The element that accepts electrons is always said to be reduced, because by accepting electrons, its positive charge goes down. However, for the transfer and capture of electrons to occur, there needs to be a medium of transfer that shuttles them from one element to the other, that medium is called an electrolyte and in the case of iron rusting, the electrolyte is water (H2O). That is why rust only occurs when iron is left exposed in moist, humid air or in water or rain and never in dry, arid conditions. When the iron gives off its electrons (oxidation), and these, in turn, are captured by oxygen atoms (reduction) in the presence of moisture (the electrolyte), it produces rust also known chemically as "hydrated iron oxide." But where does the rust form? Does it form in the air? No, it deposits itself on the surface of the metal. Since, iron now has its original electrons (which are now attached to the oxygen), plus the oxygen that bonded to its surface through water, it is now heavier than before the reaction. This was the exact opposite of how phlogiston theory tried (and failed) to explain what was happening. It just asserted that during the dephlogistication of a metal, heavy particles of air attached to and became trapped inside the metal. Of course, there was no experimental evidence for, nor explanation demonstrating how any of these assertions happened. Just a detailed description - as always - of the supposed dynamics. As you will see, these detailed descriptions of evidence-free claims are a tell-tale sign of bad science! Another chemical reaction phlogiston theory had trouble explaining was combustion. Hank Green of YouTube's CrashCourse channel fame points out the problem:
... Natural philosophers believed that chemical reactions occurred thanks to an ether - a colourless, odourless, "self-repulsive," extremely fine, and therefore hard-to-measure fluid - called phlogiston.... [Which] was released during combustion.... If you covered [a] candle with a jar, the flame would go out, because the air would become saturated with phlogiston and couldn't absorb anymore. This is exactly the opposite of how we now understand it. That the flame goes out because its used up all of the oxygen, which is necessary for a chemical reaction" Hank Green - Episode #18: The New Chemistry
Again, please notice that the old phlogiston definition was a description and not an explanation. This is hugely significant because detailed descriptions are not a litmus test for the validity of a theory. That is to say, just because something can be richly detailed does not mean such details describes reality! Or that the entity being so described is in any way related to reality. Most science fiction is incredibly detailed. Take your pick from Star Wars to the fantasy worlds of JRR Tolkien, but they are just that, fantasy. There is no land far, far away...! Hence, the dividing line between fiction and reality is not descriptive power but explanatory power! That statement is so important that it bears repeating, for it is infact, the underlying thesis of this whole blog and the litmus test for differentiating between imagination and reality: The dividing line between fiction and reality is not descriptive power, but explanatory power! This is so, for the simple reason that you can only explain something that is real, but both real and imaginary entities, ideas, and concepts can be described! Why is that? There reason is simple, yet profound. I'll let Sir Isaac Newton explain:
A man may imagine things that are false, but he can only understand things that are true, for if the things be false, the apprehension of them is not understanding" Sir Isaac Newton
Again we repeat, for this is the crux of the whole matter!
A man may imagine things that are false, but he can only understand things that are true, for if the things be false, the apprehension of them is not understanding" Sir Isaac Newton
By defining phlogiston as a fluid that was colourless, odourless, "self-repulsive," and extremely fine, and therefore hard-to-measure, its promoters were putting it beyond the boundaries of investigation. And if not investigated, with its properties verified through experimentation, data collection, observation and the forming of informed conclusions, how did or in fact, could the supporters of phlogiston theory have possibly come to know about all its properties? What were all those rich details a function of, if not of evidence derived through experimentation? The answer, as you might have already guessed is, their imaginations! And. Nothing. Else. Question. Uncomfortable question. What percentage of the many scientific things you believe in do you think are the figments of the imaginations of latter-day etherists, instead of products of experimental and observational data? Understanding that scientists often conjure up theories that have nothing to do with reality, but are a figment of their imaginations is the value of phlogiston theory to us. More than that, having come to appreciate that reality, further insights we learn from phlogiston's failure as a viable description of reality come from appreciating how vigorously its proponents fight for their bankrupt belief systems, despite mountains of mounting evidence for its debunking. Even more telling, is the nature of the last gasp efforts at salvaging a dying theory. Notably, how the last stages of a dying theory are a patchwork of targeted mini-solutions that are tacked on to the original theory in an effort to plug up its growing and widening cracks. This kind of "science," is no different from any false religion you care to name. Its proponents don't believe it due to any provable reasons, but due to irrational reasonings, which are tied to emotions - not logic! Wikipedia summarized the dominant view of phlogiston theory at the end of the 1700s as: "... A very subtle principle that vanishes in all analysis, yet it is in all bodies." Essentially, its proponents were saying I believe in it, but don't ask me for proof! That is the very definition of idolatry - and the foundation of all falsehoods! Incidentally, that line: "... A very subtle principle that vanishes in all analysis," could be the tagline for all scientific theories based on baseless ethers.
Throughout its rise and demise, different intellectuals were responsible for popularizing phlogiston theory in their respective countries or areas of influence. For instance, Johanne Juncker, Guillaume-Francois Rouelle and Giovanni Antonio Giobert. Rouelle was a prominent scientist and teacher who made unique contributions to the burgeoning science of chemistry. He used his influence to introduce phlogiston theory to France, where it quickly became accepted as conventional wisdom. His students many of whom soon became influential themselves due to their own accomplishments further promoted phlogiston theory, which only added more authority in embedding phlogiston theory as the truth that described how nature works. However, phlogiston theory had been attracting more and more detractors as its inconsistencies became a growing catalogue that became harder and harder to defend. One of Rouelle's most outstanding students was Antoine-Laurent de Lavoisier, of whom we shall next learn.
Antoine-Laurent de Lavoisier and the New Chemistry
Antoine Lavoisier (26 August 1743 - 8 May 1794) proved instrumental to the establishment of Chemistry as a true scientific discipline, one that would thereafter be as clearly distinct from alchemy as astronomy was from astrology. In attacking the perplexing problem of phlogiston theory and its many contradictions, Lavoisier decided to start from the opposite supposition: when a flame was burning he reasoned, something (phlogiston) was not being put into the surrounding air, but taken out of the air. What that something, was he did not know. But whatever it would prove to be, would be, he thought, must be - a form or type of air! He had reached that conclusion earlier as the only other entity interacting with the identified elements in these chemical reactions - including oxidation (rust) and combustion (fire) - was air.
He surmised from the experiment where a 'glass smothered out a flame' that the flame did not go out because phlogiston was saturating the air in the glass, but because a key ingredient in the limited amount of air found in the glass that was necessary for the flame to stay lit was being used up - resulting in the flame going out. His thinking was clear and logical: since ,when metals are burned they gain and not lose mass, logically, something is being added to the metal, from the air, and not the other way around. In other words he was looking for the opposite of phlogiston theory, he was looking for some sort of anti-phlogiston! All the experiments he performed were thus designed to discover this mysterious substance.
Lavoisier, is another excellent example in the cautionary tale of not accepting theories without critical analysis! Having established his name with the new Chemistry, Lavoisier abandoned the scientific method and instead invoked an Ether, this time called Caloric. Like Phlogiston theory before it, Caloric theory had no merit from its inception. However, like Aristotle, and his outsized influence on his contemporaries and centuries worth of future generations; like Ptolemy and the acceptance of his claims without a shred of empirical evidence; like Becher; like Stahl; and like Priestley; so it proved with Lavoisier as he invoked an unproven ether to describe an aspect of reality, for which he had no experimental proof. The ever-morphing ether had struck again in a new, unfamiliar guise. How many ways can the Emperor have no clothes?

Winner Takes All
The late 1700s was an exciting age, in that many scientists and amateurs alike were all trying to solve the same problems. There was much to discover and the cost of entry was cheap: the only requirement was curiosity and human ingenuity in devising experiments that could decisively prove one world view and disprove others. Around this time, one of the people trying to resolve the accumulating contradictions of phlogiston theory was Joseph Priestley, a strong believer in the validity of the phlogiston theory. Priestley, a man whose scientific experiments were largely focused on only one subject, the investigation of the different types of "airs" (what we now call gases). And, at this, he was an accomplished master, having already invented carbonated water. He also happened to be a friend of Lavoisier's, and so in 1774 , when he visited Paris to receive an award, he invited Lavoisier to join him at the occasion. Lavoisier obliged and at their meeting, was surprised to hear of a breakthrough that Priestley had made. Priestley told him about an experiment in which he was able to chemically isolate "dephlogisticated air." How? Priestley had expanded on the candle in a glass jar experiment, by adding different variables to it.
In one such variation of this experiment, Priestley put a rat in the in bell jar along with the candle and realized that both the candle died out and the rat suffocated. He soldiered on, in yet another variation, he included a small house plant under the bell jar in addition to the rat and burning candle, and placed everything in front of a window. This produced the exact opposite effect. This time the mouse continued alive and the flame didn't go out - though they were still enclosed. He was getting warmer. Priestley, the devoted advocate of phlogiston theory had managed - in his mind - to control air in such a way that the air inside the bell jar was now not filled with phlogiston, thus allowing combustion and respiration to continue - according to phlogiston theory. Of course, in reality he had discovered photosynthesis, but didn't follow this lead to its logical conclusion! To be just, he had confined the scope of his investigations to the discovering of different kinds of "airs," so perhaps his single-mindedness was an indication not of carelessness but focused diligence. Scientists had known for a while that there were different kinds of 'airs' as different chemical process were possible in it. Knowing now, that there was a way to manipulate regular air (the atmosphere) to produce a version of it that resisted the absorption of phlogiston (in reality, replacing the oxygen used up the candle with new oxygen through photosynthesis), he now attempted to isolate this never before discovered "air". He knew how long a rat left alone inside a glass bell jar should live for and this would serve as his baseline measurement. If he could find a way to increase that amount of time he would have succeeded in isolating the phlogiston resistant air, as "phlogiston" had the same effect on living animals as it did it on fire, as his earlier experiments had proved.
This he achieved in 1774, when he performed another variation of the experiment. This time he substituted a metal - mercuric oxide - for the plant and candle flame he had used previously. He then used a strong lens and light from the sun to heat up the mercuric oxide, knowing that the chemical reaction would release a type of air. To his delight it was the type of air he was looking for, because the rat stayed alive for four times longer than his baseline measurement time. Priestley, thereafter concluded that he had isolated "dephlogisticated air". That was not his only conclusion, however, and the rest proved to be a mixed bag. He correctly surmised that contrary to conventional wisdom, air was not an element, but is instead composed of many different elements, and it is these elements that allow it to produce differing effects. He had managed to isolate the one that was crucial to the chemical reactions of combustion, oxidation and respiration. However his second conclusion was not only incorrect, it was the exact opposite of reality! Here we see a point most critical to the true progress of science - the importance of matching the right theoretical framework to hard won experimental and observational data! The role of theories and their subsequent working models in the progress of science cannot be overstated! In fact, the role of science is not just to ascertain the facts, but to thereafter use those facts to arrive at the theory and model of the World that explains all the experimental and observational facts thus far catalogued. It is the theory and its correct working model, not the facts, that imparts understanding! The right facts contained in a wrong theory will always lead to incorrect conclusions, because the theory and its working model ARE THE EXPLANATION. They explain the existence of the facts; how they fit together; and what that signifies about the nature of reality. It is this significance that we call meaning. If our clear understanding of reality incorporates God, that means one thing, if it shows there is no Almighty Being behind the universe, that also has meaning - just a very different one. The settling of this question is the central issue in Science. Facts alone cannot frame meaning. Only when facts are properly housed within a coherent theory and its working model of reality, can mankind come to correct conclusions and more vitally, attain - UNDERSTANDING!

Perhaps more than any other man, Joseph Priestley, is a study in the power of a wrong mental framework to arrest progress. Perhaps more than any other man, because not only was he impeded from moving with the times by his obsolete mental framework, but he himself, provided the evidence for the verification of the new paradigm! Even in this portrait, you can see the look of resignation and stubborn stoicism in the face of defeat. Defeat by what? He didn't know. Why? He couldn't tell you. All he and his fellow believers in the ether of phlogiston could tell you was how much they believed it to be the truth, even as the evidence was mounting against it as an explanation for proper workings of "heat." He provides a firm lesson for all of us, in the power - of wrong ideas.
A Paradigm Shift
As it was, Joseph Priestley had the right facts, but the wrong mental framework, the wrong theory and model! Understanding that means we appreciate that what he called dephlogisticated air, must have been something else. The open questions were, what was it and what was its significance? Priestley only partly appreciated the first of those two questions (to the extent that he understood some of its properties) but he could hardly imagine the impact of the second. As soon as Lavoisier heard of dephlosgiticated air, he knew immediately that he had found his long searched for anti-phlogiston. "A rose by any other name...." Realizing the importance of this 'air,' he immediately left to go and try to duplicate the experiment and its results for himself. Upon attaining the same results as Priestley, he promptly named the newly discovered 'air' oxygen, publishing the results in a 1775 paper to the French Academy of Science, that laid out the the characteristics of the new 'air.' Lavoisier was diligent and disciplined and over the next twelve years, he and his team of collaborators would continue to discover and intelligently name different chemical elements. He renamed "dephlogisticated air" oxygen in 1778. Hydrogen would be named five years later in 1783, and Silicon in 1787. So useful for identifying new elements did his methodology prove to be that by 1787, he released an in depth publication on chemistry called Méthode de Nomenclature Chimique (Method of Chemical Nomenclature), essentially a textbook about his reasoning behind his new method of naming and cataloguing chemical elements and compounds. Before developing his new method, elements had names which were qualitative (descriptive), they told you nothing about what the element was and what it was composed of. For instance, hydrogen had previously been called inflammable air, lead acetate had been known as Sugar of Saturn and copper sulfate had been known as Blue Vitriol.
To understand why the new naming system was a giant leap forward, let us consider the example of copper sulfate. Here we will get into a little bit of detail but nothing you have to know or memorize. The aim of the exercise is only to show that the new nomenclature is logical and more importantly - meaningful. Atoms are neutral because they have the same number of protons and electrons. Since these particles have opposite charges the atom is neutral, but it can also exist in a charged version called an ion, when there is an imbalance between the number of electrons and protons in the atom. Ions can be either positively or negatively charged. Positively charged ions are called cations (pronounced cat ions) and negatively charged ions are called anions (an ions). When two or more different kinds of ions form a molecule, they are called polyatomic (poly = many). Sometimes that is still not enough to describe the atoms adequately, as these negatively charged ions can have more than one form. Hence, a polyatomic anion with more oxygen atoms has the suffix ate, thus when Lavoisier renamed Blue Vitriol to copper sulfate he was accurately describing the chemical composition of the substance CuSO4. He was stating the base elements that make up the compound: copper and sulfur. Additionally, the name was telling anyone who understood the naming convention, that the type of sulfur involved was the one with 4 oxygen atoms - and not the version with 3! The sole purpose for our giving this expanded illustration was to demonstrate the value of Lavoisier's new chemistry. Unlike the old 'anti-science' that was hardly distinguishable from alchemy, the new chemistry was a true Science: logical and exacting. More than being descriptive, it had the power to explain. Everything had its place and there was a place for everything. For this reason some elements in the periodic table were predicted to exist before they were discovered experimentally, because there would be a 'hole' in their place, their number, in the periodic table of elements.

Each age has its own paradigm(s), or the set of axioms, truths and falsehoods that define the mental framework of the societies that make up that period in human history. Another important aspect of Kuhn's theory of paradigm shifts, is the surprising insight throughout time, that the acceptance of an ideology as true by the scientific community is not solely based on objective criteria, but on subjective criteria, like how the scientific community as a whole views the matter. This should not be the case in a community that prides itself as being objective. Consensus is the enemy of scientific progress. We frequently hear "follow the science." As it happens scientists have biases, and these must be factored in, when accounting for which paradigm is favoured by a current generation of scientists. This is unfortunate, but true. For example: there is an odd anomoly in the Cosmic Microwave Background Radiation that seems to show that the Earth is at the center of the universe. Scientists have named it: "The Axis of Evil." Do you think that name belies the presence of a preferred Worldview by the present-day scientific community?
Quantification - the Great Divider
Another key development to chemistry becoming a science distinct from the metaphysics of alchemy, was the ability to quantify substances! Lavoisier developed a way of conducting all his experiments in sealed glass enclosures! This meant he could accurately measure both the reactants (inputs) and products (outputs) of a chemical reaction in his experiments. It was this development, more than any other that enabled scientists to refute qualitative descriptions of the alchemical arts, and establish qualitative explanations as the standard for natural phenomenon - that is, to establish Chemistry. This methodology furnished empirical evidence that chemical processes did not change the amount of mass involved in reactions, only their composition and/or appearance, and led to the law of the conservation of mass. Stated another way this law means, mass cannot be created or destroyed, it can only change form! Profound.
A major benefit of the quantification of experiments was that it left no room for confusion. For the first time, facts that could not be argued against could be established and verified independently. That was significant, because for the first time it could be proved empirically that events that seemed to be completely unrelated where actually different forms of the same phenomenon! For instance, in the winter of 1782 - 1783, Lavoisier collaborated with mathematician Pierre-Simon Laplace, with whom he had worked in a commission to establish a uniform system of weights and measures, known as the metric system, which is still the standard today. Lavoisier and Laplace used an ice calorimeter apparatus (an object used for measuring the amount of heat produced by a chemical reaction) in their new experiment. They designed it so that it could measure the heat produced by both combustion and respiration. They then measured how much carbon dioxide and heat was produced by a live guinea pig that was put inside the apparatus. Thereafter, then burned carbon to produce the same amount of carbon dioxide that was produced by the guinea pig and measured how much heat was produced. An equal amount of heat was produced, and Lavoisier an Laplace concluded - correctly - that respiration and combustion were one and the same phenomenon as they produced the same evidence profile. Incredibly, though the two processes look very different at face value, they are indeed different versions of the same essential chemical interaction. Lavoisier summed it up with the following words:
respiratory gas exchange is a combustion, like that of a candle burningAntoine Lavoisier - Wikipedia
Another prime example of the utility of this new methodology was in solving the pesky problem of why metals gained mass and hence weight, when they underwent oxidation. Phlogiston theory was being twisted into knots by its adherents in trying to explain why this was so. In the end, only chemistry could explain the dynamics behind the puzzle. For the first time apples could be compared with apples. All this new learning was being meticulously catalogued by Lavoisier, and in 1789, he published a new textbook called Traite Elementaire de Chimie (Elementary Treatise on Chemistry) that taught no alchemy, only chemistry! Nothing would ever be the same. As revolutionary as his accomplishments were in setting the foundations of chemistry, Lavoisier's ideas were not without opposition, and from non other than accomplished chemist - and friend - Joseph Priestley. His Wikipedia page noting:
While Priestley accepted parts of Lavoisier's theory, he was unprepared to assent to the major revolutions Lavoisier proposed: the overthrow of phlogiston, a chemistry based conceptually on elements and compounds, and a new chemical nomenclature. Priestley's original experiments on "dephlogisticated air" (oxygen), combustion, and water provided Lavoisier with the data he needed to construct much of his system; yet Priestley never accepted Lavoisier's new theories and continued to defend phlogiston theory for the rest of his life" Joseph Priestley - Wikipedia
Finally, the ether had been rendered obsolete. No longer would the all permeating phlogiston be used as a crutch to describe what mankind could not possibly have had an inkling of. More than that, it was Priestley's data that had initially formed the facts around which the new theory of elements and compounds was established. What was perplexing about Priestley's opposition to the founding of chemistry was that as doggedly as he defended phlogiston theory, he was defending something of which he had no knowledge, or evidence of. As many and varied as his accomplishments were, letting go of the ether proved a stretch too far for Priestley. In science, many would rather die from being wrapped in the familiar, but constricting cloak of obsolete conceptions, than be rejuvenated by the novely of fresh thinking and data-backed paradigm shifts. Again Crash Course explains:
Led by ... Priestley ... old-timers kept modifying phlogiston theory so that it could rationally account for chemical reactions without falling apart.... Well into the 1780s many chemists still believed in phlogiston - which no one had actually seen or measured - simply because it was familiarHank Green - CrashCourse
Delta - What's the Difference?
What was the difference? What was the difference between Priestley's phlogiston theory and Lavoisier's new chemistry? Methodology! Lavoisier followed the scientific method pioneered by Galileo and perfected by Newton in pursuing his science. All assessment of natural phenomena involves observation. The difference is how people interact with what they observe. No investigator can work in a mental vacuum - in the absence of an overall picture of what they think the observations represent. This is natural, and so all researchers have a working mental model as their starting point. This is always informed, by the presence of data in the end, but initially, in the absence of data, by mere assumptions and unconscious biases. These first conceptions of the nature of reality are usually incorrect. For instance when humans looked at the skies, it seemed obvious to them that the sun revolved around the earth. However nothing could have been further from the truth. How then do we arrive at that truth? We need to investigate nature. However, even these investigations are themselves harder than you might at first think. This is because to know what to investigate, is no simple matter. Over the hundreds of years of successful scientific discoveries, the catalyst to discovery has proven to be in the spotting of a paradox in what we think we already understand, and take for granted. To make real investigative progress, humans need to identify a Perspective Paradox! What is that? When we realize that how something "looks," can, in fact, not be how it really functions! The value of such perspective paradoxes is in our resolving them. Their resolution leads to a clear understanding of the workings of the deeper realities of nature that thereafter explain not only the initial paradox itself, but how our lack of understanding of nature led to it, in the first place. Thus, those who practice true science use unresolved observation as the starting point of their investigation. They observe until their observations lead to a perspective paradox. Having identified a perspective paradox, they then have a starting point for the application of the scientific method. The underpinnings of nature are counter-intuitive by definition. It is the perspective paradox that both gives us the clues as to both, what we must focus our investigations on, and what kind of experiments we must carry out to resolve it.
On the other hand, we have those for whom their observations and the mental framework they conjure up to explain them, are the conclusion. The problem with such scientists is that they want reality to fit their theory and not the theory to conform to reality. For them science is the exercise of their imagination, not an investigation into nature. That is why matching their model of the World to the data is a non-starter for them. Who needs data, when you have imagination? It is important to note that such scientists also usually carry out experiments in order to try and prove their theory(ies). However, it is how they respond to new experimental evidence that is troublesome. In the case of phlogiston theory, it was Priestley himself who discovered the evidence that would prove to be the death knell for his dear theory. Yet he still failed to come to the right conclusion. For such scientists, when new evidence contradicts their theory's stated dynamics or features, they create mini fixes, a mosaic of fractured would-be-solutions for a theoretical canvass that proves too fragile to hold them together. It never seems to occur to them, that they must abandon their old theories, for one that more accurately models the facts as borne out by empirical observations and experimental data. The key to understanding the difference between these two approaches is appreciating that one seeks to interrogate nature in trying to find out how things works. The other seeks to dictates to nature how things should work. And, when new data contradicts its old assumptions, the theorists merely double down on their position, and substitute "consensus," for now, readily available observational facts and scientific truth.
Their theories prove to be roadblocks to their success, not because they are inflexible. In the case of thinking of the ether as being responsible for the chemical processes on earth, we see that theory going through several revisions from Becher's initial hypothesis. Stahl updated Becher's theory and Priestley in turn, updated Stahl's version, so lack of flexibility in revising the theory is not the problem. With Priestley, for instance, it was not that he didn't perform experiments, or generate data, but that all such data was useless to him, as he was so limited by the parameters of his theory, that he could not imagine any other other explanation as being plausible. Why is that? He put the cart before the horse. This is dangerous for just this reason. As one early science commentator noted: "In the absense of data, the effects of heliocentrism and a geocentric model of the universe looked the same." (I'm paraphrasing.) That means at first approximation, both good and bad reasoning look like they can explain natural phenomenon adequately. That means, to truly explain the nature of reality, we have to understand the difference between explaining how things look - and how things work! What we are seeking are explanations not descriptions! As for Priestly, whatever experimental data he collected had to fit his already formulated picture of reality - and therein lies the danger! Science is not the journey of using data to confirm our intuitions about nature, but the task of using data to discover/uncover the true nature of reality. Nature, is by definition counter-intuitive! Humans are a product of the natural world and not the other way around. That is to say, nature is not the result of the human mind, but both nature and humans are the product of a far superior and loftier reality. Reality is not the product of a humand mind. Hence reality, though eventually comprehensible to humans - via Science - is nevertheless, and always will be counter-intuitive to us!
Since these two approaches to science are so inherently different, you might think that a scientist could never employ both in his lifetime. You would be wrong. In fact, many eminent scientists, have swung from one approach to the other without hesitation, as was the case with our esteemed Mr. Antoine Lavoisier, himself. However such an approach will always fail, because nature is not only counter-intuitive, but since it is not the product of human minds, but of a far loftier Divine mind, it must by definition fall outside of the scope of all of mankind's thoughts and intiutions! This should be self-evident. Hving said that, it doesn't mean mankind cannot grasp the truths of nature. Quite the opposite: we can understand them quite readily - once they have been discovered. The difficulty is not in understanding discovered truths but in unearthing them in the first place. The only scientific bridge between human ignorance and scientific fact is the scientific method. Hence, when humans take the approach of merely looking at nature and then coming up with theories to explain what they "see," without going through the process of experiment and data collection, without going through the scientific method, they have always been proven wrong. In the end. This is so because without experimentation and data collection, humans do not even know what they are looking at:
... Natural philosophers believed that chemical reactions occurred thanks to an ether - a colourless, odourless, "self-repulsive," extremely fine, and therefore hard-to-measure fluid - called phlogiston" Hank Green - CrashCourse
We now know that such a view was proved invalid, but let us carefully examine it to see if it EVER had any merit! It lists five characteristics of phlogiston: it is 1) an ether 2) colourless 3) odourless 4) self-repulsive 5) extremely fine and 6) it is a fluid. Question: were these assertions conclusions or assumptions? They were of course, blind assumptions, as no experiments were carried out to establish any of these supposed features of phlogiston. Let us be clear, Priestley did carry out many experiments, and in fact, Lavoisier's early successes in the field were based on experimental results obtained by Priestley! The point, however is that those experiments did not benefit Priestley, as he didn't use them to come to any valid conclusions and the conclusions that he did reach were not based on the evidence resulting from his experimental data - but rather, blind guesswork! That was the tragedy of his efforts. In contrast:
Lavoisier's system was based largely on the quantitative concept that mass is neither created nor destroyed in chemical reactions (i.e., the conservation of mass). By contrast, Priestley preferred to observe qualitative changes in heat, color, and particularly volume. His experiments tested 'airs' for 'their solubility in water, their power of supporting or extinguishing flame, whether they were respirable, how they behaved with acid and alkaline air, and with nitric oxide and inflammable air, and lastly how they were affected by the electric spark'" Joseph Priestley Article - Wikipedia
Experiment & Data Gathering are the Key!
In essence, Priestley only looked at matters in a subjective way. Where he promoted phlogiston theory someone else could have put forth a wholly different explanation - as in fact did happen. The base problem was that the foundations of phlogiston theory were based purely on the whims of its inventors. The theory was not an attempt to explain how nature works, for which you would need facts. Instead phlogiston was described in a way to make it beyond any form of perception, and thus render it untestable! Thereafter, the untestable theory was used to explain what can be seen. The scientific revolution started by Galilei and perfected by Newton insisted on experimentation and facts! The secret to Lavoisier's breakthrough's is that he put aside unproven hypothesis and focused on experiment and facts. Out of all the tools scientists have come up with over the centuries, the worst is the ether. It is used as an universal band-aid to explain what has not been experimentally confirmed. If we look back at how it has been used from the times of Aristotle down to Priestley, it becomes obvious that it is the universal go to panacea whenever humans do not know what is happening. It is the blank canvas that by definition can accommodate any hypothesis. But the ether's greatest strength - its endless malleability - is also its biggest weakness! Due to its blank canvas appeal, when experiments are conducted and facts gathered, it is easily dispelled. For lazy scientist's who are drawn to them, ether's are useful because they allow them to come up with detailed descriptions of phenomenon they have never gathered facts for. There is no difference between a theory based on an ether and imaginative storytelling and baseless assumptions. However, scientific progress, if you have been paying attention, is anything but a set of empty assumptions. As for forming scientific in the absence of data, when humans look at a system or structure in nature, they never estimate its true complexity correctly. They never accurately guess the number of actual moving parts it takes to create such a system. The problem with ethers, is that in initially trying to describe how the whole system operates, they commit, through their descriptions, to how many moving parts the system has and how they relate to each other. Once the experiments are conducted, and the numbers start to come in, they then spend their time trying to reconcile the facts to their dataless assumptions. As hard as it is to believe, even though the ether has been shown to always lead scientists down the wrong path, a path that initially seems to be satisfactory as it provides imgainative details that seems to merge function with appearance, in the end, explanations that depend on an ether, fail, due to unavoidable flaw. The lack of empirical evidence.
Galileo insisted on experiment as the only means to knowledge. So how, we may ask do others pluck theories from the air based on ethers that have no empirical evidence or observations behind them? The question is relevant because Joseph Priestley would not be the last scientist to depend on an ether to try and explain the workings of nature! We now turn our attention back to Lavoisier. In doing so, we will see why ethers are the very opposite of Science. Instead of acquiring data through experimentation and then forming testable ideas to see if our theories hold; ethers put the cart before the horse by postulating how some phenomenon works, and then, thereafter, collecting data and trying to force it to reflect the dataless picture the believers in ethers, originally. Nothing in life works like that!
Caloric Theory and the Ever Changing Disguises of Ether: The Great Pretender
Success at last! With the old guard of Phlogiston believers dying out, and the new breed of students and scientists being firm believers in the foundations of the new Chemistry, Antoine Lavoisier, had managed to shift the world's thinking away from the unfounded and now falsified theory that the natural world was formed from different combinations of only four (originally claimed) elements - earth, water, fire and air. As we have shown, this ancient framework of the nature of the world had gone through many alterations over thousands of years of adding, subtracting and refining the definition of this or that element, but in its most basic form, the world was said to be made of only four elements and the variety of substances was due to the different combinations of these four elements.
Ethers Substitute for Facts in Times of Ignorance
Some of the modifications were substituting the three Terras for air and fire as proposed by Becher. Later modifications to Becher's own modifications were made by his student Stahl, who substituted a fire-like Ether known as Phlogiston to replace Terra Pinguis. Phlogiston theory never matched empirical evidence. As more and more data kept mounting against it, its proponents desperately tried to make more and more modifications in futile attempts to save a false and dying theory. Unfortunately, this trend in science - of stubbornly sticking to assumptions even after they have been proven to be demonstrably false - is one we will continue to witness. For his part, Joseph Priestley went to his grave still refusing to accept the new theory of elements and the validity of Oxygen as one such element - even though he was the one that discovered it! Let that sink in.
Knowledge, however, cannot be held ransom by ignorance. In the place of ignorance, Lavoisier introduced the concept that the world was made of different, unique ELEMENTS and he and his colleagues managed to isolate, identify and classify 55 such elements in what would prove to be the forerunner of today's periodic table! The periodic table of natural elements has 94 such substances. As of November 2016, the International Union of Pure and Applied Chemistry recognized 118 - with 94 being natural and 24 being produced synthetically in nuclear reactions. That means Lavoisier and his colleagues managed to identify a full 58% of the 94 natural elements in their pioneering efforts! What an achievement. The validity of his new method had been proven beyond all doubt, as was the superiority of the principles of the scientific method such as: formulating testable theories, the value of experiment and deriving conclusions from data-based observations - as opposed to coming up with never ending variable descriptions of some unconfirmed ether. Additionally, Lavoisier was able to discern and propose a new theory that, elements do not change their mass when they undergo phase transitions due to adding or subtracting heat. In other words, water was water, whether it was in its solid, liquid or gaseous state. It didn't lose its essence or fundamental nature in the transition. This meant he had separated what things were made of from the process of applying heat to them. This seems logical to us now, but in the 1700s it was a radical idea. That's because prior to Lavoisier, men like Becher, Stahl and Priestley had all confused the transfer of heat with the dynamics of fire - combustion. We have previously alluded to the many variations of, 'the world is made up of four elements' camp. We will now show why Lavoisier's proposal was a radical departure from these theories by considering how it differed to the last version of these theories.
Phlogiston was proposed as a theory of combustion - of why things burned. It said that all materials were a combination of phlogiston and their calx, or ash. For things to be combustible they had to have phlogiston. In their thinking, phlogiston was not fire itself, rather the flame was the manifestation of the release of phlogiston. Don't ask. In this mental framework, phlogiston was turned from Terra Pinguis into Terra Lapida, substances that like ash or calx could not burn. Moreover, phlogiston itself had different properties, depending on the kind of substance it was combined with. So phlogiston acted differently with metals than it did with organic matter such as trees. Once organic matter had been burned to a crisp - by releasing all its phlogiston - it's ash could not be burned any further, and it was a different material to what it was initially. In this way, the very nature of things was intimately tied to whether or not they had any phlogiston! Lavoisier said no! Water was water, whether it was in its solid, liquid or gaseous form - irrespective of how its interactions with heat changed it from from state to the next. More than that, its mass did not change in those phase transitions, which would be the case if phlogiston was being transferred in and out as the substancec changed from one state to another. Lavoisier's insight into the true identity of chemical elements was nothing short of profound!
You might be surprised then, to find out that when faced with his next scientific challenge: explaining how those phase changes of matter occurred, Lavoisier depended not on the scientific method that had served him so well, but that he himself invoked a new kind of ether, a 'fluid,' which he named "Caloric." In addition to its initial definition as a fluid, Caloric also came to be thought of as a weightless gas that had the same properties as its liquid form and could penetrate solids and liquids by flowing in and out of them. As is usually the case with ethers, it was thought that the quantity of Caloric was constant throughout the universe. This feature of ethers is a constant feature of wherever they are invoked because their proposers never have a way of explaining how more ether would appaer over time. Thus from beginning to end there must always, but always, be the same amount of ether in the universe. As it applied to heat, because heat was said to be a fluid, a material substance, it was said to fall under the law of conservation of mass - which applies to all material substances. This in turn should have meant that heat could neither be created nor destroyed!
The Almost Irrestible Pull of Ethers
Where phlogiston was erroneously used to describe the nature of fire and chemical processes, Caloric was now being used to try and explain the yet misunderstood phenomenon of how heat brings about phase changes (how it changes substances from their solid to liquid to gaseous states of matter). In effect, while he correctly identified oxygen and its role in combustion, when it came to explaining heat and its effects, Lavoisier reverted back to alchemical superstition. Where his predecessors had made Phlogiston the essence of heat, he made Caloric the essence of heat! Substituting one ether for another to explain the same effect is not science, nor is it progress! In Lavoisier making heat an ether, suddenly heat could not be created or destroyed, only transferred from one substance into another. Just like Caloric heated substances indicated the presence of caloric, its absence meant the opposite - the substances would be cold. You might be starting to see a pattern, as this is the third time we have come across scientists using an ether to try and explain something they do not yet understand. It usually takes around 3 instances for a simple pattern to be recognized, and the utilization of ethers for this purpose is as simple as it gets! When we review how the ether is always employed, we see that it is never used to explain or describe the things that humans understand - but always it is used to form a detailed description of what we do not yet understand! Thereafter, when the scientific method has been utilized to achieve a true breakthrough in knowledge, it never confirms the existence of the supposed ether whose dynamics were used to explained what we observed, but rather the reverse is true! Such breakthroughs always, without exception, debunk the earlier, often detailed, descriptions that supposedly explained how the ether worked. Instead of verifying the ether, such breakthroughs establish that forces and particles are responsible for generating the effects we observe! This is why the study of such phenomena is called Physics, and not Magic or Metaphysics!
All of scientific history proves that the explanation for all phenomena in the universe can always be reduced to forces and particles and never to metaphysical ethers! It is only when we are still in the dark about either the identity of the forces, or particles - or both - that humans invoke ethers to explain dynamics that are not yet understood. By the way, a sub-premise for this whole episode is that false religion and false science are one and the same! You will not be surprised then to discover that false religion uses the very same tactic and that the omnipresent (existing in all places at the same time), triune Catholic god is the grandest of all ethers!
Codename: Operation Regression
It is Hard to Stick to the Rigours of the Scientific Method
Though, he had just comprehensively proven that his friend, Joseph Priestley's formulations based on phlogiston were wrong, Lavoisier inexplicably, chose to abandon the scientific method that had worked so efficiently in his advantage, and followed the age-old disproven mental framework of using an ether to undergird a new theory. Where his own understanding ran short he filled the missing knowledge, not with a commitment to further research and experimentation but with a new type of ether that could magically explain how, what he didn't understand, worked. I say magically, because - of course - there was no experimental data or observational proof that such an ether existed, or worked the way Lavoisier asserted - as there never is, for those who advance ethers as solutions. In fact, even as he was giving detailed descriptions of how Caloric theory supposedly worked, Lavoisier was proving that, actually, he didn't understand the nature of heat at all! The Wikipedia page on Caloric Theory states that:
Chemists of the time believed in the self-repulsion of heat particles as a fundamental force thereby making the great fluid elasticity of caloric ... an anomalous property which Lavoisier could not explain to his detractors" Wikipedia
You will agree, I am sure, that if someone cannot explain something, they do not understand it. Anomalous means: inconsistent with or deviating from what is usual, normal, or expected (Merriam-Webster). Deviating from a general rule, method, or analogy; abnormal; irregular (Wordnik). It deviated from the norm, because in proposing it Lavoisier deviated from the scientific method that had allowed him to discover and furnish proof for the existence of so many chemical elements. Why could Lavoisier describe Caloric theory in great detail, but couldn't explain it? Because Caloric theory was a figment of his imagination and had nothing to do with the reality of how heat works. The Wikipedia entry for Caloric defines it thus:
The caloric theory is an obsolete scientific theory that heat consists of a self-repellent fluid called caloric that flows from hotter bodies to colder bodiesWikipedia
The man who had managed to identify 55 chemical elements through meticulous research and experimental proof, was now asserting that a new type of ether had properties such as being a "self-repellent fluid," that could penetrate substances. He even described exactly how it was supposed to work: "'... by pushing ice particles apart.'" Whenever scientists learn something new about nature, they never learn all that there was to learn. There is always a new area of investigation that the new paradigm opens up for further research. When that happens the proper scientific attitude is to mark such new areas out for further inquiry, not to blindly ascribe certain unfounded properties to them through the use of an ether.
How the Scientific Method is Practiced
The best example of sticking to such discipline is Sir Isaac Newton. When he published his revolutionary understanding of motion and the laws of gravity, he explained only as much as he understood - and nothing more. While he was troubled by the fact that he had not identified the agency of gravitational force, he refrained from proposing an unsubstantiated ether to fill that void. As ever with him, he left further scientific investigation for later generations whenever he could not finish such investigations himself. This was the case with the true nature of light, and of how it interacts with gravity. Sir Isaac Newton, humbly realized the true cumulative nature of knowledge, and how each discovery unearths two more puzzles to be solved. That means people with finite lifespans can never discover all there is to know. Actually, the truth is even more startling as Newton himself pointed to:
To explain all nature is too difficult a task for any one man or even for any one age. Tis much better to do a little with certainty & leave the rest for others that come after than to explain all things by conjecture without making sure of any thing" Sir Isaac Newton
And ...
I shall not mingle conjectures with certainties
And again ...
Thus far I have explained the phenomena of the heavens and of our sea by the force of gravity, but I have not yet assigned a cause to gravitySir Isaac Newton
And yet again ...
I have not been able to discover the cause of those properties of gravity from phenomena, and I frame no hypotheses; for whatever is not deduced from the phenomena is to be called a hypothesis, and hypotheses, whether metaphysical or physical, whether of occult qualities or mechanical, have no place in experimental philosophy" Sir Isaac Newton
It Takes True Humility to be a Real Scientist
The man who discovered the laws of gravity was not so proud that he couldn't admit to the limitations of his knowledge. Whilst others attempted to go further than what had been proven scientifically, he never indulged such tendencies! Notice how he explicitly states that any theory that is not based on the phenomenon in question is defined as a hypothesis, and further that he does not frame (or construct) any such theories! He then adds that, such conduct is not a part of the "experimental philosophy" i.e. the scientific method! Below he contrasts his behaviour as a true scientist with those who indulge in empty, unfounded hypotheses:
You sometimes speak of gravity as essential and inherent to matter. Pray do not ascribe that notion to me, for the cause of gravity is what I do not pretend to know, and therefore would take more time to consider of itSir Isaac Newton
Humility. Newton, master scientist that he was, understood intrinsically, that ethers were impediments to and not vehicles of true understanding and he vehemently objected to their use. Consider the following quote:
Against filling the Heavens with fluid Mediums, unless they be exceeding[ly] rare, a great Objection arises from the regular and very lasting Motions of the Planets and Comets in all manner of Courses through the Heavens" Sir Isaac Newton
In current English, that is: if the Heavens were filled with "fluid Mediums" (ethers), the planets and comets would not be able to move in all their different directions through the heavens, as they regularly, and always, do. This insight from Newton, highlights the crux of the matter: ethers and forces and particles cannot co-exist. This applies not only to their co-existing in the same space (which is obvious), but also more broadly, for fundamental reasons. If ethers existed, the laws governing them would be fundamentally different from the ones that regulate forces and particles. Forces and particles are part of Physics. No one knows what the laws governing ethers would look like, as no ethers have ever been found in nature. In stark contrast, Lavoisier conjured up an ether and described its dynamical effects. Of course there was no experimental proof for any of these assertions about what caloric was, OR how it was supposed to work. By now, you should be starting to realize that ethers are invoked, only, when scientists cannot identify the mechanisms: force; and associated particle, that perform an observed function in nature!
The Scientific Method Progresses Human Knowledge - Ethers Arrest it!
It was obvious to all that matter had at least three phases: solid, liquid and gaseous. What was unknown in Lavoisier's time was how heat brought these transitions about. As for ethers, invoking them unders such circumstances never helps the situation, for the following reason. The historical utility of ethers to scientists - how they've actually been used - lies only in assuming and describing in detail, the dynamics or functions of entities, the scientists do not yet understand! In other words, throughout the history of science, ethers have never been used to explain the parts of the universe that humans actually UNDERSTAND! Only the parts we do not! Ethers are thus synonymous with ignorance - a form of a sort of self-assured non-knowledge. Never have they proved to be some form of prescient knowledge itself! However, none of the scientists who invoke them, ever admit as much. This is why it has usually proven very difficult indeed for he old guard to move with the times, when actual knowledge - does finally arrive. Instead, even in the face of now overwhelming evidence to the contrary, they will stubbornly hold fast to their cherished, and now sadly outdated views. This sad feature of science shows up time and time again within the scientific community. Do yourself a favour and keep your eyes peeled for current day examples.
For now, back to caloric. When Lavoisier made his claims about Caloric being a new kind of ether, he had no experimental basis for such an assertion, and if there is no experimental evidence, one can ask the question: how then did he know the characteristics of such an ether? This sad episode in Lavoisier's career highlights another truth about the ether: there is no such thing as a new type of ether - only a new type of ignorance about the nature of reality. The ether always serves the same purpose, to describe phenomenon without the benefit of experiment! For that reason, the fundamental nature of all proposed ethers are essentially the same, no matter what entity or process they are meant to be explaining. Spot the similarities between Lavoisier's ether and Priestley's. In both the ether is described as being lighter than air - essentially weightless. This means it cannot be measured and thus falsified, as it always falls just outside the limitations of mankind's current measurement abilities. Another similarity is the need for the ether to be colourless and odourless. Again, effectively meaning it cannot be detected. Lastly, both ethers were self-repulsive, giving their proposers a sliding scale of possibilities to describe how any unknown effect worked by drawing on the dynamics of the ether. Another common feature of ethers is that they are claimed to be omnipresent (occupying all space and time), as was the case with Phlogiston, which was thought to fill the air and could thus be absorbed by plants, and animals through respiration and was also to be found in water, clouds and lighting. Another example of an omnipresent ether was Aristotle's heavenly mediums. Historically, ethers have been universally applicable, their context does not matter. In the case of Aristotle the ether was a 'stand in' for all sorts of things including gravity. It was the substance from which his celestial spheres were made, these inturn, held all other orbiting celestial bodies in place. He even described some of its features (by "local motion" he meant spinning in place, like the relative motion of the Sun in our solar systemm, or the earth spinning on its axis):
Aether differed from the four terrestrial elements; it was incapable of motion of quality or motion of quantity. Aether was only capable of local motion. Aether naturally moved in circles, and had no contrary, or unnatural, motion. Aristotle also noted that celestial spheres made of aether held the stars and planets. The idea of aethereal spheres moving with natural circular motion led to Aristotle's explanation of the observed orbits of stars and planets in perfectly circular motion" Wikipedia - Article on Aristotle
In the case of Becher, the ether was a stand-in for chemical reactions; in the case of Priestley, it was a stand in for oxygen, in the case of Lavoisier it was a substitute for our lack of understanding of how heat is produced and how it transfers from one entity to another. Do you see the pattern? Hank Green, says the following about the rampant practice of scientists invoking ethers in the 1700s: "...Ether was the explanation for many unknown phenomenon in the 18th century, and there were a lot of conflicting ether theories." As Newton so pointedly stated:
We are to admit no more causes of natural things than such as are both true and sufficient to explain their appearancesSir Isaac Newton
The Difference Between Empty Hypotheses & Bold or Educated Guesses
To put it simply, we can "admit" no law, cause or effect as true if it has not been proven to be true by experiment! Secondly, the admitted cause must be an explanation of the phenomenon, and not a description. That is how the scientific method separates fact from fiction. A theory can only be considered factual once it has been proven true by experiment. It is not science to choose to abide by the principles of the scientific method when it's convenient, as in the case of Lavoisier disproving Priestley, and then summarily do an about turn and disregard all of the same scientific principles - by invoking ethers - when we come face to face with a new mystery, for which, we as yet - do not have the facts. Remember Newton's advice: "I frame no hypotheses." Having said that we have to make an important disctinction, a bold or educated guess is very different from hypothesizing with ethers and presenting our resulting imagination as a feature of reality. To show that guesses are required in the exercise of scietific thought we turn to two masters fo the game. First, Richard Feynman outlines for us how the scientific method, actually works - in practice:
First you guess. Don't laugh, this is the most important step. Then you compute the consequences. Compare the consequences to experience. If it disagrees with experience, the guess is wrong. In that simple statement is the key to science. It doesn't matter how beautiful your guess is or how smart you are or what your name is. If it disagrees with experience, it's wrong. That's all there is to it" Richard P Feynman
Newton adds his two cents:
No great discovery was ever made without a bold guessSir Isaac Newton
Nature uses simple laws to perform all its wonders. That is, causes have effects and every effect has its own cause(s). Ethers are a way of insinuating effect(s) without identifying a verifiable CAUSE. True, their proposers assign assumed causes to them, but they do so without the theory proving experimentally that the observed effects are indeed functions of said causes - and that is the problem. Without substantiating your claims through experiment and evidence, your assumptions are nothing but empty speculation. If the cause has not been reduced to fact by experimental proof, it cannot be classified as a cause, since the theory has not been validated! The scientific method, as informally alluded to by Feynman above, is more formally defined by the following steps: 1) observation/question 2) research 3) form a theory 4) test theory through experiment 5) analyze experimental data and lastly 6) form and report evidence-based conclusions! This is a repeating loop as understanding one level of nature only serves to unearth questions about ever deeper levels and so the cycle of investigation continues. Forever. Scientists who rely on ethers, seemingly follow the first three steps, but once they have formed a theory, they effectively abort the rest of the scientific method. Thereafter, their theory or hypothesis serves as their conclusion! While - like Priestley - they may experiment, they do so not to test their assumptions, against the results of the experiments to see whether they validate, or nullify their initial guess. This unfortunate mindset, means they thereafter, view their experimental data through the lense of the theory and not the other way around: as a litmus test for its validity - or debunking. The theory should be tested against the evidence. Without this step, whatever we are doing is not in harmony with the scietific method. Instead, such scientists try to mold the data to be in line with their assumptions, no matter how complicated this makes the resulting framework. Nature, however, is not like that. In a word, it is - simple. Once again, Newton:
Nature does nothing in vain when less will serve; for Nature is pleased with simplicity and affects not the pomp of superfluous causesSir Isaac Newton
This proven fact about nature serves to illustrate the reality that out universe is one predicated on work. That is, to create an effect work must be done. That necessitates a force to be spent and all forces are allied to corresponding particles. To put it simply, all forces are mediated by particles, so-called force carriers! What does that mean? The insightful Sabine Hossenfelder explains:
... If there is a force between two particles, like say, a positively charged proton and a negatively charged electron, then you can understand that force as the exchange of another particle between them. For the case of electromagnetism, that exchange particle is the photon, the quantum of light" Sabine Hossenfelder
Evidence is the Key
What all this means is, there is no room for magic in the universe! For something to happen, something must be responsible, that is what the expression: "forces are mediated by particles" means. In the presence of knowledge about both the forces at play and an understanding of the particles responsible for producing them, there is never any recourse to ethers of any kind! Scientists never mention them. Only in the absence of such knowledge and understanding, do scientists repeatedly fall back on the endlessly employed and verified substitute of true knowledge - the ether. Thereafter the invoking scientist has the freedom to define his 'new' ether by any terms he wishes and attribute to it all sorts of wonderful and unproven dynamics. All this, without the need to identify the particles responsible for mediating the actions produced, as the actions are said to be manifestations of the ubiquitous ether. Which, as we have learned, is odourless, colourless, invisible and hard to detect - in other words beyond all ability to be detected. You might think, then, that the foremost reason to invoke ethers are their easy applicability in masking unknown variables. You would be wrong - as we will soon see.
Observations are More Important After not Before Experiments
If we are to understand how scientific discovery works, it is crucial for us to follow the story of phlogiston to its bitter end. For instance, while it had been noted that if you put a glass over a burning candle, the flame goes out, no one could prove why that was so. We now know. It is because fire needs oxygen, and when you cover it with a glass, it quickly runs out of oxygen and the flame dies. But why would it go out if the theory of phlogiston was correct, since the supply of the combustible phlogiston was to be found in the candle itself? In that case shouldn't the flame go out only after all the phlogiston in the candle was used up? The answer, according to the supporters of phlogiston theory was that as the fire burned, it released phlogiston into the limited air within the glass and once that air was saturated with phlogiston, no more phlogiston could be released from within the candle and the flame would die out. See the difficulty? Before Chemistry was distinguished from Alchemy, and established as a proper science, there was no way to separate fact from fiction, subjective descriptions from objective explanations. Since both explanations didn't use data, it was impossible to verify which one was true!
If an explanation does not provide valid experimental data such as numbers that can be measured and verified, it is a description and not an explanation. Always. Explanations based on phlogiston, were readily accepted as they seemed to explain what was being observed. We should be noticing a pattern by now. Such erroneous conceptions of reality are designed to appeal to the visual senses, by forming theories that only take the appearance of how something seems to work, into account. And not the empirical facts, about how it - actually - works. The truth is, such theories are always formed independetly of any experimental data. On the other hand: the scientific method only weighs the observations made after an experiment has been conducted. As such, both approaches take into account how things "look," but ethers put the horse before the cart, by looking and deciding first, and then experimenting; while the scientific method, experiements first, and only then, "observes" the results of the experiment, in order to come to an evidence-backed conclusion. It's as if those who invoke ethers believe an abundance of descriptive detail is equal to explaining how reality works. Of course, the two are very different things. Children's books often involve very richly detailed storytelling. However, in the end they are fables which have no bearing on reality. Both real and false stories can be richly detailed, but only real phenomena can be explained. This distinction is vital, as explanation and understanding, versus description and myth, are properties found only in reality and never in imagination!
It is vital because all scientific investigation is a quest to distinguish between a wrong and right interpretation of observed phenomenon, and initially, to the eye both types of models of the World: the ether derived model; and the experimentally derived model appear equivalent. And it is for that reason that ether-derived versions of reality initially catch on with the public. When we look at historical civilizations, we see a continual trend for humans to give unfounded explanations for phenomenon they do not understand - a tendency for them to "hypotheses ... fingo" or frame hypotheses. No civilization ever says I don't know what a rainbow is. Instead, they come up with fanciful myths - usually involving false gods - about why aspects of nature are as they are! And therein lies the true value of the scientific method - it separates fact from fiction! It is experiment that separates the true from the false! As Feynman says: "The test of all knowledge is experiment." For instance, before solid proof was furnished, claims that the earth stood still and everything orbited around it and the counter-theory, that the earth turned on its own axis and revolved around the sun, were visually EQUIVALENT. You couldn't tell which was true and which was false based solely on how "things looked." Even today, we all still refer to "sunrises" and "sunsets!" Only detailed evidence from Brahe, Kepler and finally Galileo settled the matter for good. The same can be said for all other ancient mysteries that have now been solved. Do you remember the redox reactions and how they cause rust in iron? Without experiment, the phlogiston theory seemed just as plausible as Antoine Lavoisier's explanation, which only careful experiment proved to be correct. As it turns out, understanding redox reactions is important for far more than just appreciating how rust works. For now, though, let us concentrate on how understanding the role of oxygen in combustion led mankind to solving our next puzzles - understanding what heat and temperature were; and how they worked!
